But can the use, and exploitation, of the legal options the patent law provides be anti-competitive ?
On Oct 31, 2024 the European Commission fined drugmaker Teva € 462,5 mn for alleged (a) misuse of the patent system and (b) spread of misleading information about a competing product, so as to hinder its market entry.
The case is about the multiple sclerosis drug glatiramer acetate, which is a L-Ala-L-Glu-L-Lys-L-Tyr-Polymerisate. Teva has approved the drug in the EU in 2001 under the brand Copaxone. The basic patents expired in 2015. Still, Copaxone is among the most widely used drugs for the treatment of multiple sclerosis.
As a result of a) and b), the Commission concluded that Teva had abused a dominant position in the markets in Belgium, Czechia, Germany, Italy, the Netherlands, Poland and Spain.
In this article, we will not comment on the second allegation (according to which Teva had implemented a systematic disparagement campaign against a generic), we will analyze the first allegation.
While the respective decision is not published yet, in a press release, the Commission gave some details regarding the alleged misuse of the patent system. According to the Commission’s findings (inserts and added),
(i) Teva filed multiple divisional patent applications in a staggered way, creating a web of secondary patents aroundCopaxone focusing on the manufacturing process and the dosing regimen of glatiramer acetate. Rivals challenged these patents [by way of opposition] to clear the way to the market,
(ii) pending review by the EPO, Teva started enforcing these patents against competitors to obtain interim injunctions,
(iii) When the patents seemed likely to be revoked, Teva strategically withdrew them, to avoid a formal invalidity ruling, which would have set a precedent threating other divisional patents to fall like dominos,
(iv) by doing so, Teva forced competitors to repeatedly start new lengthy legal challenges.
(v) This tactic allowed Teva to artificially prolong legal uncertainty over its patents and, potentially, hinder the entry of competing glatiramer acetate medicines.
Indeed, our search revealed that Teva has applied a very extensive patent strategy, with alone in Europe at least 60 patent applications in 43 patent families.
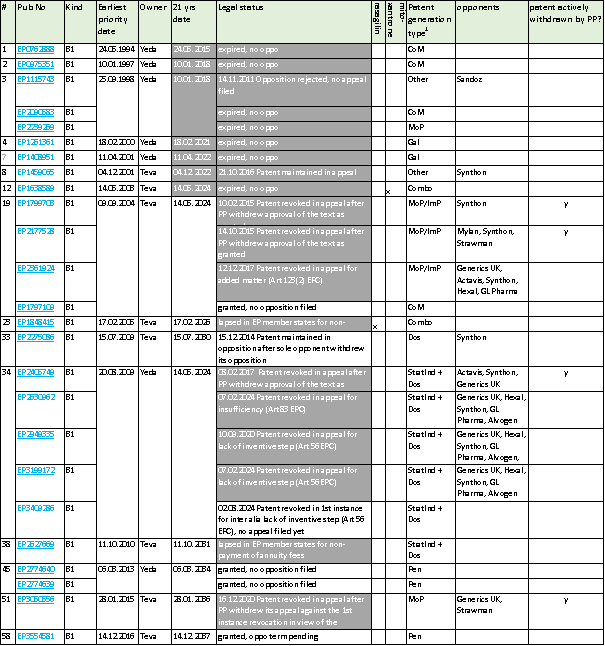
1CoM = composition of matter, Dos = dosage or dosage regimen; Gal = galenics; StratInd = stratified indication, combo = combination, MoP = method of production, ImP = Intermediate product, GlaOpt = other product with Glatiramer optionally, Pen = Injection Pen, PCoM = Packaging of Composition of Matter)
25 granted EP patents emanated therefrom, out of which 11 were challenged by way of opposition – all finally revoked but 3 (one maintained, one revoked in 1st instance yet appeal term pending, and one where the opposition term is still pending). Quite a few are lapsed already, and, currently, only 6 granted patents remain, our of which one is in the opposition term, and another one was revoked in opposition, appeal term pending.
Yet what Teva did was legal, and fully within the framework of what the European Patent convention allows. And for a patent practitioner, Teva’s filings strategy is far from surprising – it is pretty much within what originators typically do (while Teva obviously knows both sides of the table, the generic and the originator side).
One creative aspect is that in 2014, Teva approved a new formulation with 40 mg/mL dosage instead of 20 mg/mL, because patents on the original formulation were about to expired. 70% of patients shifted to the higher dosage formulation, for which Teva had filed patent applications in 2009 already.
As regards the Commissions’s objection that “when the patents seemed likely to be revoked, Teva strategically withdrew them, to avoid a formal invalidity ruling, which would have set a precedent threating other divisional patents to fall like dominos”, see the last column of the above table to see to see that Teva acted in such manner in four cases.
In fact, this type of accusation is not a new one. One memorable case is the UK High court case Fujifilm/Samsunf vs. Abbvie, regarding 2nd generation patents for Humira, in which Justice Carr acccepted a contention made AbbVie's strategy would consist of “dragging out proceedings for as long as possible, causing maximum expense and inconvenience to its opponents, and then throwing in the towel just before its patents are scrutinised by the court, whilst covering the same subject matter with further divisionals.” This was not a competiton law case though, yet it was merely at stake whether invalidity proceedings in the UK should be continued in view of the fact that AbbVie had withdrawn the patent in corresponding EPO opposition proceedings.
However, can the use, and exploitation, of the legal options the patent law provides be considered anti-competitive ? Article 102 of the Treaty on the Functioning of the European Unio.n (TFEU) prohibits the abuse of a dominant position. But, in order to execute a patent strategy as Teva did for Copaxone, one does not need market dominance. A deep pocket (and probably good attorneys) is all that is required.
However, what could have taken the commission over is the combination of the alleged “systematic disparagement campaign” with the said, lets call it, “extensive” patent strategy. We will learn more soon, because Teva has, in a press release of 31 Oct 2024, its discontent with the decision, and announced that it will appeal it.